On August 18, 2022, the Centers for Medicare and Medicaid Services (CMS) released a roadmap to support healthcare providers with preparing for the eventual end of the COVID-19 public health emergency (PHE) declaration. CMS also published a series of fact sheets summarizing the status of Medicare Blanket waivers and flexibilities by provider type as well as flexibilities applicable to the Medicaid providers and stakeholders.
In its announcement, CMS expressed concern that the continued PHE flexibilities could contribute to further decline in patient, resident, and client safety beyond what has already been observed. As a result, the agency is cautiously working to balance ongoing PHE needs while conveying more urgency for providers to prepare for the eventual end of the PHE flexibilities and waivers.
CMS has already ended certain flexibilities and waivers. The agency could phase out other flexibilities as it prepares to let the PHE declaration lapse. This means that providers and health plans should act now to assess flexibilities and waivers in use and develop a plan to transition to post-PHE environment.
Phase-out of COVID-19 Flexibilities
During the COVID-19 PHE, CMS has utilized Medicare and Medicaid waivers and flexibilities extensively. For example, Medicare has not enforced certain federal requirements during this time to allow hospitals to utilize off-site locations to screen and treat patients when needed as well as to minimize certain reporting requirements. The agency’s flexibilities also have accelerated adoption of telehealth and audio only services, particularly for behavioral health services.
Medicare and Medicaid providers across the states utilized PHE flexibilities to varying degrees, in part depending on experiences in individual communities, capacity, and other provider specific factors. Additionally, over the course of the PHE health plan and provider staffing and workflows have changed dramatically. This means health plans and providers will need a tailored plan to support the transition to the post PHE environment.
HMA’s experts are working with hospitals, health systems, clinics, and other providers as well as health plans on the steps they need to take now to prepare for multiple transitions. Our experts identified six immediate steps that Medicare and Medicaid plans and providers can be undertaking now to ensure they can effectively return to normal operations, including:
- Review performance on the patient and clinician safety metrics cited in the new CMS resources. In instances where providers have gaps and suboptimal safety and quality outcomes they will need assistance developing and implementing mitigation and quality improvement plans.
- Utilize CMS’ tailored fact sheets to identify specific flexibilities and waivers in use now and the “normal” federal regulations that will be in effect once the PHE lapses. This assessment should include the blanket waivers and provider specific flexibilities, including:
- Flexibilities around the requirements and timing for practitioner training
- Expansion of allowable sites of service that permitted more expansive use of telehealth and virtual services as well as screening and treatment provided at alternative sites
- Relaxation of federal requirements pertaining to surge capacity protocols
- Flexibilities for staffing requirements, including medical records departments, nursing facilities, among others
- Waiver of requirement for hospitals to submit occupational mix surveys and to have a utilization review plan with a UR committee focused on services furnished to Medicare and Medicaid enrollees
- Applicable of the Extreme and Uncontrollable Circumstances Policy in Medicare’s Shared Savings Program and use of other flexibilities for MSSP Accountable Care Organizations (ACOs)
- Non enforcement of certain physician self-referral laws
- Waiver of numerous reporting requirements including those pertaining to verbal orders, discharge planning, HEDIS and STARs measure reporting, among others
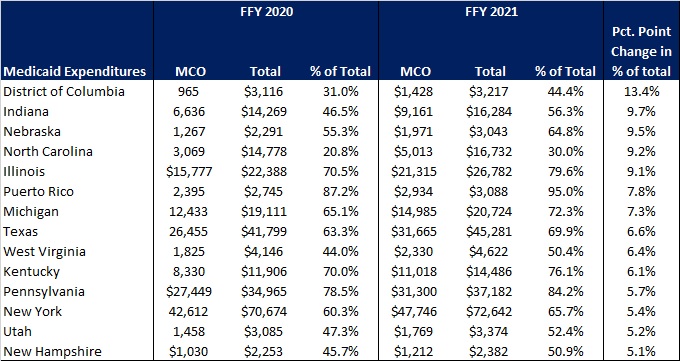
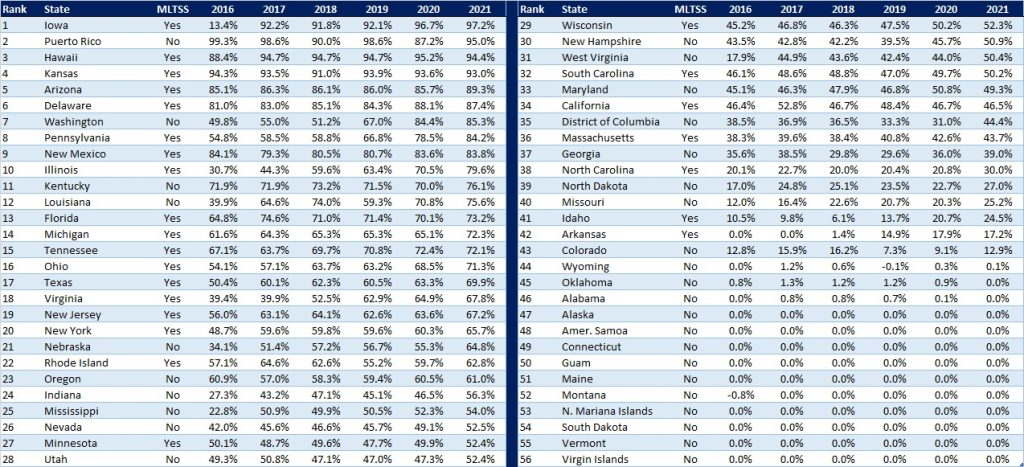
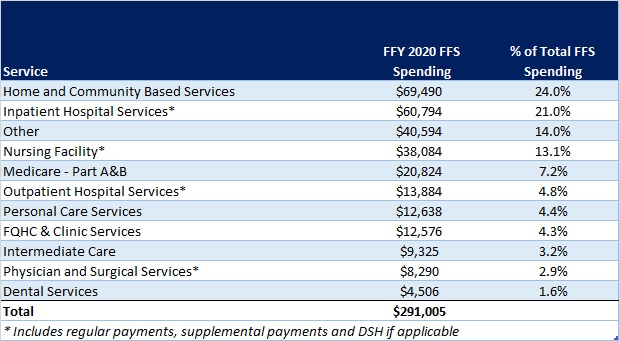
- Project impact of ending Medicaid’s continuous coverage policy and support individuals with actions they may need to take at the end of the PHE. Once the PHE ends, Medicaid’s enhanced federal funding for states and continuous coverage policy will end. HMA is working with health plans, providers and other stakeholders to project how this change will impact the enrollment and payer mix on state and local levels. Additionally, patients and their caregivers will need support from plans, providers, and consumer groups to ensure they renew their coverage or transition to other coverage programs when needed. HMA’s experts have written extensively about our work to support the Medicaid unwinding activities in two blog posts – The PHE is continuing—what’s next for Medicaid? and How stakeholders can prepare now for unwinding of Medicaid public health emergency continuous eligibility.
- Develop a plan to transition from “PHE” to “post-PHE” expectations that is informed by the assessment of flexibilities in use. Key components of the plan include:
- Anticipated resource needs to reflect changes in staffing and workflows during the PHE
- Articulation of specific compliance procedures and regular reporting requirements that will resume and the process for this transition
- Develop training and education opportunities for staff that may be new or need refresher on normal policies and procedures as well as timeframes for making these changes
- Update budgets projections to account for changes in reimbursement rates for certain services post-PHE. Certain reimbursement amounts and payment methodologies will change post-PHE, such as payment for administering the COVID-19 vaccine in a Medicare patient’s home among other changes. Providers will need to project the financial impact of these payment changes and update coding and billing manuals and procedures where applicable.
- Build strategies to sustain changes to care models implemented during the COVID-19 PHE while also addressing health disparities. Some providers and facilities adopted care models and modified existing ones during the PHE that may have improved patient outcomes and experiences, maximized expertise of practitioners, and improved value-based care. For example, some providers have embraced the Medicare Hospital Without Walls Initiative and will need to assess their options as some of those flexibilities are phased out. Other federal opportunities have newly emerged during the pandemic, such as the Rural Emergency Hospital designation and pending changes to the Medicare Shared Savings Program (MSSP).
What’s Next
The COVID-19 PHE declaration next expires on October 12, 2022. While a renewal of the PHE declaration is possible into early 2023, providers should be using this time to prepare for resumption of normal policies and procedures.
The expiration of PHE flexibilities and waivers are not, however, happening in a vacuum. Providers need to make this transition amidst a dynamic healthcare sector with high expectations for continuous improvement in quality, patient experiences, and value. During this transitional period HMA’s experts are working with health plans and providers to develop or revisit strategic plans and investments to refocus attention on improving models of care and value-based payment approaches, including strategies that will help mitigate health disparities.
For questions, please contact our experts below.