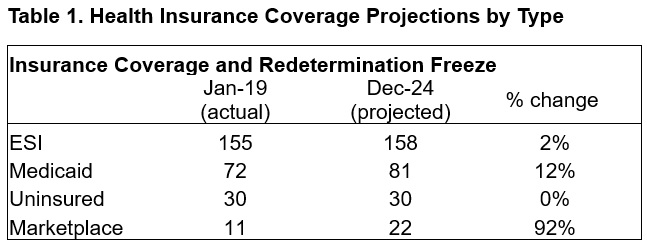
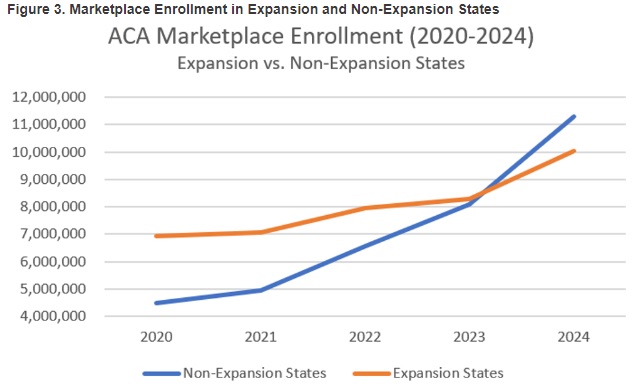
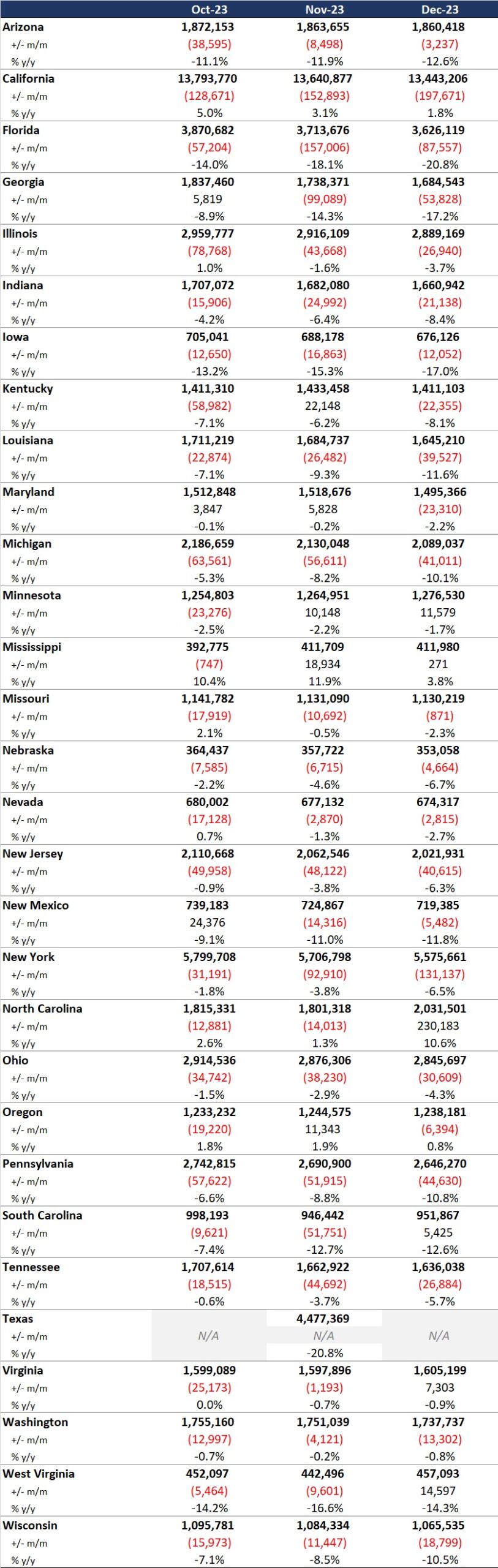
Our second In Focus section provides a refresher on the Medicaid and Children’s Health Insurance Program managed care access, finance, and quality proposed rule that the Centers for Medicare & Medicaid Services (CMS) published in May 2023. As Health Management Associates, Inc. (HMA), has noted, the final rule is expected to be published later this month. If finalized as proposed, several provisions in the rule will signal the start of a new era of accountability and transparency for the Medicaid program.
The policy changes are expected to fall into the following major categories: in lieu of services (ILOS), the Medicaid and CHIP Quality Rating System (MAC QRS), medical loss ratios (MLRs), network adequacy, and state directed payments (SDPs). These revised policies will affect Medicaid coverage and reimbursement for years to come. Following is a summary of the proposed policy changes to watch for in the final rule.
ILOS
CMS has proposed to expand upon and codify the sub-regulatory guidance around ILOS outlined in State Medicaid Director Letter #23-001. The letter advised state that they have the option to use the ILOS authority in Medicaid managed care programs to reduce health disparities and address unmet health-related social needs, such as housing instability and nutrition insecurity. The final rule would expand upon and codify that guidance.
For example, although the ILOS proposal adds reporting requirements and guardrails to address fiscal accountability, the proposed rule also noted that the substitution of an ILOS for a state plan service or setting should be cost-effective but does not need to meet budget neutrality requirements. States are also permitted to specify that an ILOS can be an immediate or longer-term substitute for a state plan service or setting.
MAC QRS
CMS has proposed a MAC QRS framework that includes: (1) mandatory quality measures, (2) a quality rating methodology, and (3) a mandatory website display format. State Medicaid agencies and managed care organizations (MCOs) will be required to adopt and implement the MAC QRS framework that CMS develops or adopt and implement an alternative but equivalent managed care quality rating system. CMS will update the mandatory measure set at least every two years. Any planned modifications to measures will be announced publicly through a call letter or similar guidance, with measures based on: (1) value in choosing an MCO; (2) alignment with other CMS programs; (3) the relationship to enrollee experience, access, health outcomes, quality of care, MCO administration, or health equity; (4) MCO performance; (5) data availability; and (6) scientific acceptability.
State Medicaid agencies will be required to collect from MCOs the data necessary to calculate ratings for each measure and ensure that all data collected are validated. In addition, state Medicaid agencies will be expected to calculate and issue ratings to each MCO for each measure.
Lastly, state websites will be required to contain the following elements: (1) clear information that is understandable and usable for navigating the website itself; (2) interactive features that allow users to tailor specific information, such as formulary, provider directory, and ratings based on their entered data; (3) standardized information so that users can compare MCOs; (4) information that promotes beneficiary understanding of and trust in the displayed ratings, such as data collection timeframes and validation confirmation; and (5) access to Medicaid and CHIP enrollment and eligibility information, either directly on the website or through external resources.
MLRs
CMS has proposed three areas for revision to its existing MLR standards, which require MCOs to submit annual MLR reports to states, which, in turn, must provide CMS with an annual summary of those reports. Areas for revision include: (1) requirements for clinical or quality improvement standards for provider incentive arrangements, (2) prohibited administrative costs in quality improvement activity (QIA) reporting, and (3) additional requirements for expense allocation methodology reporting.
With regard to provider incentive arrangements, CMS proposes to require that contracts between MCOs and providers: (1) have a defined performance period that can be tied to the applicable MLR reporting period(s), (2) include well-defined quality improvement or performance metrics that the provider must meet to receive the incentive payment, and (3) specify a dollar amount that can be clearly linked to successful completion of these metrics as well as a date of payment. MCOs would be required to maintain documentation that supports these arrangements beyond attestations.
In terms of QIA reporting, CMS proposes to explicitly prohibit MCOs from including indirect or overhead expenses when reporting QIA costs in the MLR. CMS also intends to add requirements regarding how MCOs can allocate expenses for the purpose of calculating the MLR by requiring MCOs to offer a detailed description of their methodology.
Network Adequacy
CMS has proposed a range of new network adequacy requirements intended to improve timely access to care for managed care enrollees. Those related to appointment wait time standards and secret shopper surveys are among the most prominent.
For appointment wait time standards, CMS proposes that state Medicaid agencies develop and enforce wait times associated with routine appointments for four types of services: (1) outpatient mental health and substance use disorder (SUD) for adults and children, (2) primary care for adults and children, (3) obstetrics and gynecology (OB/GYN), and (4) an additional service type determined by each state Medicaid agency using an evidence-based approach. The maximum wait times must be no longer than 10 business days for routine outpatient mental health and SUD appointments and no more than 15 business days for routine primary care and OB/GYN appointments. State Medicaid agencies could impose stricter wait time standards but not more lax ones. The wait time standard for the fourth service type will be determined at the state level.
State Medicaid agencies also will be required to engage an independent entity to conduct annual secret shopper surveys to validate MCO compliance with appointment wait time standards and the accuracy of provider directories to identify errors, as well as providers that do not offer appointments. For an MCO to be compliant with the wait time standards, as assessed through the secret shopper surveys, it would need to demonstrate a rate of appointment availability that meets the wait time standards at least 90 percent of the time.
SDPs
CMS has proposed several important changes to the requirements governing the use of SDPs, strengthening both the accountability required of and flexibility afforded to states. For example, CMS proposes to require that provider payment levels for inpatient and outpatient hospital services, nursing facility services, and the professional services at an academic medical center not exceed the average commercial rate. Furthermore, states would be required to condition SDPs upon the delivery of services within a contract rating period and prohibited from using post-payment reconciliation processes.
With regard to flexibility, CMS proposes to remove unnecessary regulatory barriers to support the use of SDPs by states to implement value-based payment arrangements and include non-network providers in SDPs. The proposal also permits states to implement, without prior approval, minimum fee schedules in Medicaid consistent with Medicare provider rates.
What’s Next
CMS is expected to publish the final rule in April. In addition, CMS plans to publish a separate final rule addressing new regulations pertaining to access to care, which will have equally significant impacts on states, MCOs, and providers. If you have questions about how HMA can support your efforts related to the managed care final rule’s implications and the context of other federal regulations for states, MCOs, or providers, contact our featured experts.