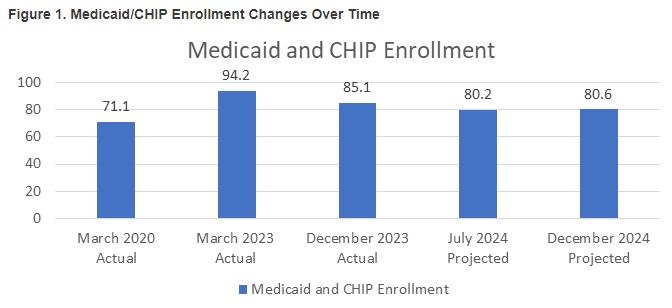
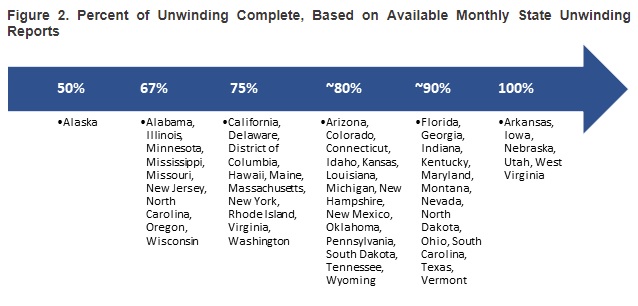
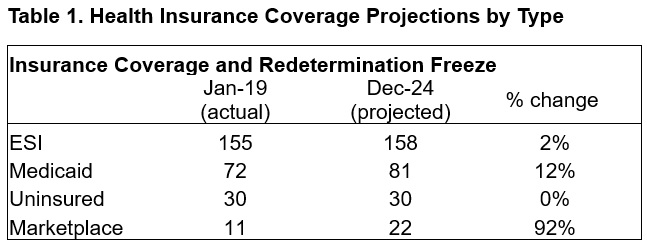
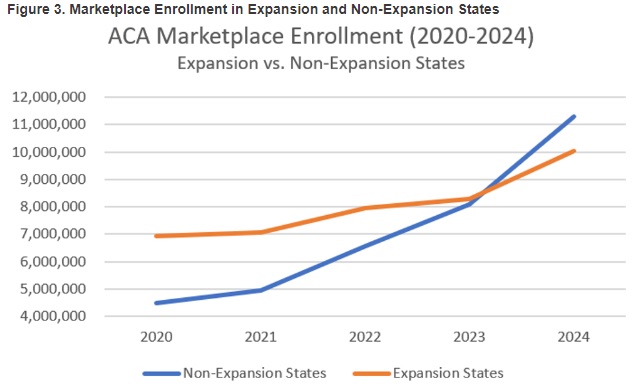
This week, our In Focus section reviews the year-end legislative package congressional leaders announced as part of the stopgap funding to prevent a government shutdown. The package, which was unveiled December 18, 2024, would extend expiring Medicaid and Medicare policies, reauthorize health and human services programs, and extend federal funding for discretionary programs through March 14, 2025. The existing temporary funding measure expires December 20, 2024.
Following is a summary of several major healthcare policies that, if approved, will inform the shifting federal policy landscape and state and local programs in 2025.
Pharmacy Benefit Managers
The healthcare package includes policies that reflect several years of increased scrutiny on pharmacy benefit managers (PBMs), including:
- Prohibiting PBMs from charging a Medicaid managed care organization more for a drug than the amount that a PBM pays a pharmacy (i.e., spread pricing)
- Requiring consistency and additional transparency in contracts between Part D plans and PBMs
- Prohibiting Medicare Part D plans from linking payments to drug list prices
- Adding report requirements for PBMs
Medicaid Policies and Programs
The legislative text includes 13 separate sections that address Medicaid policies, including extensions on expiring policies, establishment of new programs, and plans to codify certain other policies related to Medicaid eligibility and renewals. These policy changes include:
- Medicaid Disproportionate Share Hospital (DSH) allotment: Eliminates reductions for fiscal year (FY) 2025; delays the effective date of the two remaining years of Medicaid DSH allotment reductions until January 1, 2027; and changes the definition of the Medicaid shortfall component of the Medicaid DSH cap to include costs and payments for patients who have Medicaid as their primary source of coverage and for patients who are dually eligible for Medicare and Medicaid.
- Home and community-based services (HCBS) waiver: Establishes a three-year, five-state Medicaid HCBS waiver program, which would allow states to cover these services for individuals who need them but do not meet the current statutory requirement of needing “institutional level of care.” States will have an opportunity to apply for planning grants.
- Services for juveniles leaving public institutions: Delays by 12 months the requirement that state Medicaid programs provide screenings, diagnostic services, and targeted case management services for eligible juveniles within 30 days of their scheduled date of release from a public institution following adjudication.
Medicare Payments
The compromise package also increases the Medicare Physician Fee Schedule conversion factor by 2.5 percent in 2025 to partially offset a 2.83 percent cut that the Centers for Medicare & Medicaid Services (CMS) finalized in November. Providers consider this a short-term fix, however, and Congress, provider advocates, and other interested parties are engaged in discussions about making broader changes to Medicare physician pay in 2025.
Notably, the agreement includes a payment policy consistent with a bill that the House of Representatives passed earlier this year—the Lower Cost More Transparency Act—to provide enhanced information about payment differentials between off‐campus outpatient departments and other outpatient facilities. The provision requires each off-campus outpatient department to obtain and bill for services under a unique national provider identifier.
Other notable Medicare policies include:
- Telehealth: Extends Medicare telehealth flexibilities through December 31, 2026; establishes special rules for telehealth services provided by Federally Qualified Health Centers and Rural Health Clinics for prospective payment and all-inclusive rates; adds modifiers for telehealth services provided incident-to other services and those offered via contracts with virtual platform vendors; expands services that can be provided via telehealth; and enhances tracking of telehealth use
- Payment extensions: Extends the Medicare low-volume hospital payment adjustment and Medicare-dependent hospital program through December 31, 2025; Medicare ground ambulance add-on payments through December 31, 2026; incentive payments for advanced alternative payment models through payment year 2027 at an adjusted amount of 3.53 percent; and Qualifying Participant eligibility thresholds in effect for performance year 2023 through payment year 2027
- Hospital at-home program: Extends the Acute Hospital Care at Home initiative through December 31, 2029
- Part D: Prohibits cost sharing for generic drugs for Part D beneficiaries who are eligible for the low-income subsidy
- Provider directories: Requires Medicare Advantage plans to maintain accurate provider directories on a public website beginning in plan year 2027
- Screening: Adds multi-cancer early detection screening tests as a covered benefit beginning in 2029
- Home infusion: Allows coverage of home infusion treatments by classifying certain approved infusion treatments as Durable Medical Equipment (DME)
Other Notable Provisions
- Reauthorizes and revises the Second Chance Reauthorization Act of 2024, including allowing substance use disorder (SUD) services to be provided through the State and Local Reentry Demonstration Projects program
- Reauthorizes and modernizes several aspects of child welfare programs
- Provides mandatory funding for community health centers and the National Health Service Corps through FY2026, the Teaching Health Center Graduate Medical Education Program through FY2029, and the Special Diabetes Programs (SDP) for Type I diabetes and the SDP for Indians through FY2026
- Reauthorizes through FY 2029 the SUPPORT for Patients and Communities Act, which includes a range of mental health and SUD prevention, treatment, and recovery programs
- Reauthorizes Older Americans Act programs
- Reauthorizes several programs and authorities related to preparedness and response through FY2026, including the Public Health Emergency Preparedness Program and the Hospital Preparedness Program
What’s Next
Funding for the federal government expires December 20, 2024. Congress will need to approve another temporary measure to avert a government shutdown. The length and scope of such an extension remains under discussion, though the current continuing resolution would push the funding deadline into the first few months of the incoming Trump Administration and new Congress. Healthcare stakeholders, including payers, state and local governments, providers, and community organizations, should continue to monitor the congressional negotiations and be prepared to analyze the impact of legislation that Congress ultimately approves.
Connect with Us
Health Management Associates, Inc. (HMA) experts will continue analyzing the implications of the funding and policy updates in the December 18 package and ongoing congressional discussions to reach an agreement. HMA’s experts have the depth of knowledge, experience, and subject matter expertise to assist organizations with navigating these changes and the impact for health and health adjacent sectors. Please contact Laura Pence and Andrea Maresca to connect with our experts.